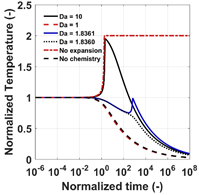
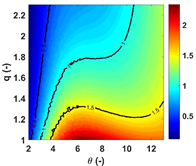
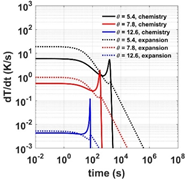
In this study, a 1-step and a 2-step globalized model are used to investigate the competition between chemical kinetics and volumetric expansion. Parametric studies have been conducted to better understand how the critical Damköhler numbers (Dacr) are related to characteristic chemical time scales: the induction and excitation times. Results show that a gas particle can be cooled down to only 30% of the original temperature and ignite due to strong chemical heat release. For the 1-step model, the contour for Dacr exhibits bifurcation behaviors for Dacr > 1. Results for the 2-step model demonstrates a ‘saddle-like’ structure of Dacr in the q1-q2 space (qi is the reduced activation energy of the ith chemical step) which disappears as q increases. The Dacr contours are symmetric about the q1 = q2 line. The increase of chemical endothermicity within the induction period leads to an increase of Dacr.