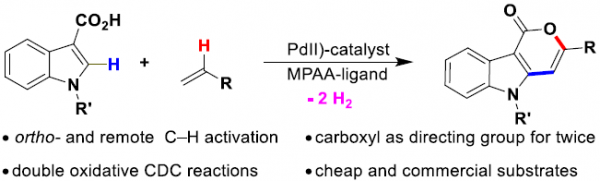
Carboxylate-Assisted Pd(II)-Catalyzed ortho-C–H and Remote C–H Activation: Economical Synthesis of Pyrano[4,3-b]Indol-1(5H)-ones
2019
期刊
Organic Letters
- 卷 21
- 期 8
- 页码 2847-2850
- American Chemical Society (ACS)
- ISSN: 1523-7060
- DOI: 10.1021/acs.orglett.9b00851
