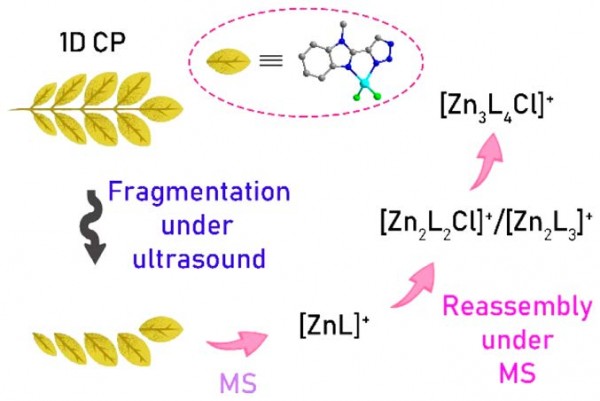
Fragmentation of a One-Dimensional Zinc Coordination Polymer and Partial Reassembly Evidenced by Mass Spectrometry
2019
期刊
Crystal Growth & Design
- 卷 19
- 期 11
- 页码 6801-6805
- American Chemical Society (ACS)
- ISSN: 1528-7483
- DOI: 10.1021/acs.cgd.9b01247
